
Stories

Agata Smogorzewska: Exploring DNA Repair for Disease Prevention and Treatment
“It was a memorable time for me,” remembers Agata Smogorzewska. “I was in the chemistry department. I don’t remember what I was doing there. I saw an advertisement on the wall to apply for a research semester at one of the Department of Energy laboratories. After learning more about this opportunity, I asked one of my college mentors whether they knew Judy Campisi, whose work interested me, and he did!”
Smogorzewska’s mentor helped facilitate a network connection with Campisi, who agreed to welcome Smogorzewska in her lab after she was accepted into the program. “I spent a semester at the Lawrence Berkeley National Laboratory, and my project was to clone human telomerase, which was obviously very ambitious, and I failed, of course. If I had cloned it, I would have been famous.”
This experience was the first of many steps that led Smogorzewska to The Rockefeller University, where she is now a tenured professor and head of the Laboratory of Genome Maintenance. Her start in science and research began in Warsaw, Poland, where she was encouraged to follow a career as a physician-scientist by her family of chemists and biologists. Her mother and father worked as an immunologist and physician, respectively, and she has fond memories of spending time with her mother in the lab as a child and even attending her mother’s doctoral thesis defense. Through her father, she was exposed to books about medicine, where she read about different diseases and patients’ personal stories. Smogorzewska was hooked. “There were many unknowns and a bit of a mystery with why they developed certain diseases,” she remembers thinking. In high school, Smogorzewska became even more fascinated by cell division. “Just watching cells divide—going through mitosis—and realizing that not everything about the process was known; this piqued my interest.”
After graduating high school, Smogorzewska continued her education in the United States, where she attended the University of Southern California and received a bachelor’s in molecular biology and biochemistry. She had three lab experiences and loved them all. “That’s when I got interested in how cells overcome normal regulatory pathways and become cancerous. The first experience I had was in a bacteriology lab. I had a project over the summer to understand how plasmids, circular pieces of DNA, were transferred between different bacteria. I didn’t discover much, but it was a terrific experience, and it introduced me to molecular biology and cloning,” Smogorzewska recalls. “I had a wonderful mentor, Dr. Deonier, who loved teaching. It was a small lab, so I had a lot of attention and encouragement to explore other research areas. I also took a graduate-level course with him, which shaped how I think about science.”
After college, Smogorzewska earned her M.D./Ph.D. from the Tri-Institutional M.D./Ph.D. program, conducting her Ph.D. research at The Rockefeller University in the lab of Titia de Lange (1995 Rita Allen Scholar). During her rotation, Smogorzewska cloned TRF2, a gene coding for a protein that binds to the end of the chromosome—or telomere—protecting the ends and regulating telomere length. “My interest in DNA repair started with my graduate work, being interested in how cells protect their telomeres,” Smogorzewska explains. “What happens when the telomeres become unprotected is that they start fusing with other telomeres; they start repairing themselves, which for a telomere is an inappropriate behavior. I thought exploring how DNA is repaired in dividing cells would be interesting. During replication, when our DNA is duplicated to give rise to new cells, many events are happening simultaneously. How you coordinate different events during this time of high activity in the cell and how the cell does it without making mistakes, still is a major question in biology.”
These questions led Smogorzewska to complete her postdoctoral fellowship with Stephen J. Elledge at Harvard Medical School, where she identified FANCI, one of the genes associated with Fanconi anemia. The Elledge lab allowed her to work collaboratively on projects and lead her own work, allowing her to start her independent laboratory as an assistant professor. By the time Smogorzewska joined Rockefeller, Arlene Auerbach, who had created a registry for Fanconi anemia (FA) patients in the ’80s, was retiring. Smogorzewska used samples collected by Dr. Auerbach to identify and study new genes mutated in patients with Fanconi anemia.
Her current work at Rockefeller continues to focus on understanding how our genomes are kept intact throughout our lives and what happens when they become unstable, leading to cancer development. She continues to study Fanconi anemia patients who often present with bone marrow failure and developmental abnormalities in childhood, and are at high risk of developing head and neck, esophageal, and anogenital cancers as adolescents and young adults.
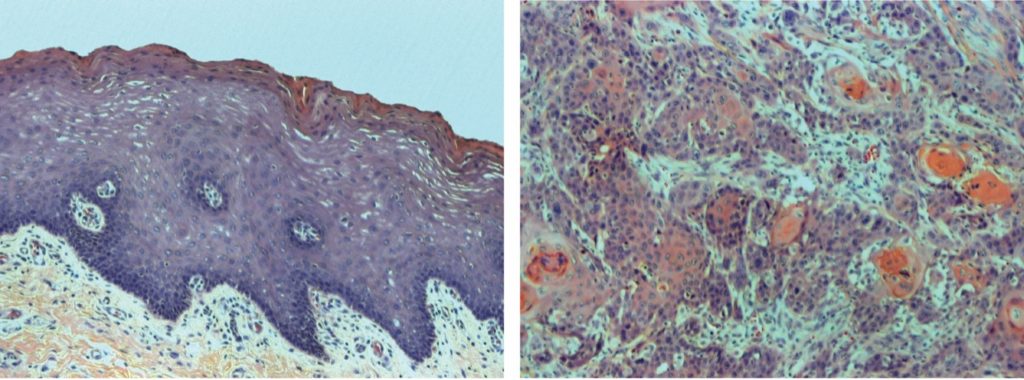
“It was striking that the biggest challenge that Fanconi anemia patients were facing after they received a bone marrow transplant that solved the blood problems was the development of these horrible, horrible, aggressive squamous cell carcinomas,” says Smogorzewska. “Unless the tumors are identified very early, patients do not live long after the cancer diagnosis because the tumors are challenging to treat. There was very little known about how the tumors developed or how to treat them.”
For the past ten years, Smogorzewska’s group at Rockefeller has been collecting patient tissues and working collaboratively with countless researchers, families, physicians, and the Fanconi Cancer Foundation. Throughout their hard work, Smogorzewska’s group discovered that the tumors were driven by genome instability—pieces of chromosomes scrambled with many pieces missing and others having too many copies. “Based on our work, we also believe that the lack of DNA repair in these tumors allows them to undergo epithelial-to-mesenchymal transition, a process which helps cancer cells spread locally and metastasize to distant sites. This, in addition to the many genetic changes, makes the tumors more aggressive, and we’re still striving to understand how this happens.”
In addition to furthering understanding the underlying biology of Fanconi anemia, Smogorzewska’s work has been crucial for understanding how sporadic head and neck cancers develop in the general population through the same biological mechanisms and pathways that occur in Fanconi anemia patients. She hypothesizes that exposure to toxins, such as aldehydes from tobacco smoke, pollution, and alcohol, overwhelms the Fanconi anemia pathway, leading to poor repair of DNA and the introduction of genetic mutations that lead to head and neck cancers.
“I believe this is the most significant of our most recent studies. It’s a critical cancer for a rare disease, and we discovered connections between the mechanism of tumorigenesis in the rare hereditary cancer that develops in Fanconi anemia patients and the sporadic head and neck cancer. Our findings are already galvanizing additional work into understanding these cancers including biomarker development to simplify clinical trials which we are embarking on.”
Smogorzewska became a Rita Allen Scholar in 2010 after joining Rockefeller in 2009. “It was one of my first grants, and it was the second one that I had to present and defend in person in a chalk talk,” she remembers. “So, that was an interesting experience because one gets immediate feedback about their ideas. The grant supported the bread and butter of what we were doing then, but had enough breadth to follow different paths when we discovered something new. And, looking back at my publications from that time, I cited the Rita Allen Foundation on many papers that came out from the lab. It was a fast-moving and competitive time—we were identifying genes and studying their function to figure out how they worked in DNA repair. We also built models to study what happens when these genes are absent. The majority of the genes that we study have human disease associations, so that gives insight into how DNA repair contributes to health and disease.”
Here, Smogorzewska discusses why her research is important, what challenges she has faced in her career, and what she likes to do outside of the lab.
If you’re speaking to a more general audience, how can you convey the importance of understanding rare diseases, like Fanconi anemia, and DNA damage and repair pathways?
Even though people may think that DNA damage and repair pathways are complex and impenetrable, they are fundamental to the biology of all our cells. Some of them are even essential, meaning there wouldn’t be life without them. The majority of cancers increase with age, and some of it is due to the DNA repair pathways waning, resulting in an increase in genetic mutation rates. Much of the critical DNA damage during our lifetime is an endogenous type of DNA damage, meaning it comes from within our cells. Suppose you understand where the damage comes from and the pathways that repair such DNA damage. In that case, you might increase the cell’s ability to deal with some of this endogenous DNA damage and possibly prevent some of the cancers that plague us. As in any fundamental biology study, we must understand how these processes work before intervening.
What are some of the biggest challenges you’ve encountered in your career?
Studying a rare disease is a challenge. Part of it is because you have a limited number of patients you can study or ask to participate in your research. Collaborations are essential, but our academic structure only sometimes recognizes that collaborations are necessary. Some might need more credit because they are perceived as relying on their collaborators as if their ideas were not also independently developed. I believe that there is a challenge in that. The other challenge continues to be funding for rare diseases. There’s a lot that can be done, and rare diseases can be excellent models for sporadic diseases in the general population, but that is not always apparent. This means that a funder might not recognize how the potential findings will apply to understanding a problem that is pertinent to all of us.
What sparks your interest outside the lab?
I’m very fond of classical music, so I love going to the opera and to concerts. These days, I occasionally get to do that. I’m busy with my two kids and dealing with their schedules. So, currently, I’m the best taxi mom. I also like to bake and cook when I have time. You probably get that from many scientists because it’s like being in the lab, but better—you get to eat what you make!