Stories

Vivianne Tawfik: Clinically Informed Basic Science
Physician-scientists are the bridge between bench and bedside. What they observe in their patients becomes the subject of lab research, and what they learn from their lab research ideally translates into improved patient care.
Stanford pain specialist Vivianne Tawfik, M.D., Ph.D., is a prime example of a physician-scientist whose robust link between these realms has both elevated patient care and delivered new insights about the molecular underpinnings of pain. “My clinical practice and my research lab are really tightly aligned. I like to call it ‘clinically informed basic science,’” Tawfik says.
A fascination with pharmacology initially drew her to pain research. As a McGill University student in Montreal in the 1990s, her first job was assisting pain specialist Mark Ware, M.D., M.Sc., with a comprehensive review of the scientific literature on cannabinoids and pain.
“That summer I spent hours and hours photocopying papers and making a reference list of all the papers I could find,” she recalls. “It made me really interested in the concept of applying pharmacology to chronic pain, which affects one in three Americans. That’s more than 100 million people. I ultimately decided to apply to M.D.-Ph.D. programs, because my research interest was drug mechanisms, but I also really wanted to care for patients.”
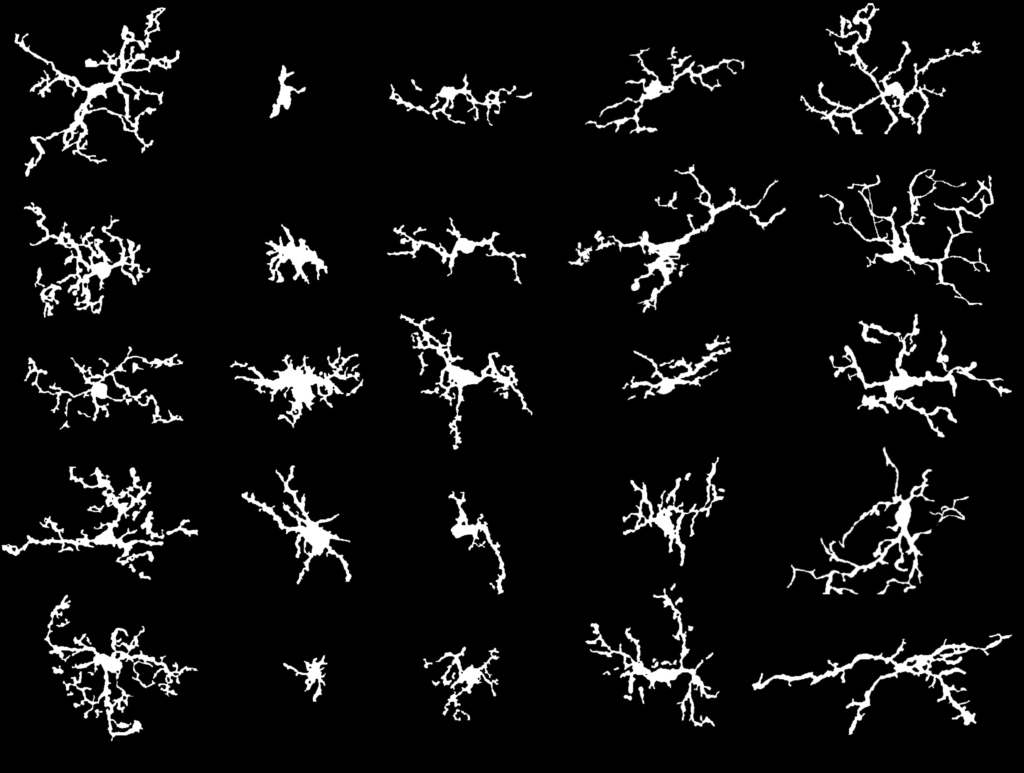
It was while in pursuit of her dual degree at Dartmouth Medical School that she homed in on glial cells’ role in pain. Once thought to simply provide a scaffold for neurons in the spinal cord and brain, glial cells are now known to have a far more sophisticated repertoire. Astrocytes, for example, orchestrate neuronal function across millions of synapses—an ability that may contribute to pain by strengthening the communication from neuron to neuron. And microglia are immune cells, so they become a double-edged sword: on one side, protection against viruses or bacteria, but on the other, a flood of inflammatory molecules, which creates another pathway to heightened neuronal activity—and pain.
With the guidance of her thesis advisor, Joyce DeLeo, Ph.D., she discovered that microglia and astrocytes play key roles in post-injury pain responses and opioid tolerance. And during her postdoctoral training with Gregory Scherrer, Ph.D., she teased out a new understanding of certain opioid receptors—a widely cited finding with significant implications for the study of opioid tolerance.
Since joining the faculty at Stanford, Tawfik has simultaneously made major insights into pain mechanisms in her lab and led an outpatient clinical practice on campus. One of her specialties is complex regional pain syndrome, a type of chronic pain that occurs in the limbs after an injury, trauma, or surgery. Her patients’ experiences inform her research.
“All of the mouse models we use essentially replicate the phenotypes my patients are dealing with,” she says. “That way, what we discover in the lab is directly relevant to their care.”
Recently, for example, Tawfik observed that her patients received substantial pain relief from the antimalarial drug hydroxychloroquine, which can reduce inflammation. She took that insight to members of her lab, who investigated the drug further in a mouse model to test its effects. “Lo and behold, the mice got so much better,” she recalls. “The finding completely changed how I prescribed hydroxychloroquine. I used to consider it a last resort, but after that, I gave it to patients much earlier because it seemed to have real potential to alter the course of their condition with few side effects. And that’s turned out to be true.”
Many of the mechanisms behind pain remain elusive, which is why Tawfik believes the support for basic research like that provided by the Rita Allen Foundation Scholars Award in Pain, which she received in 2019, is so crucial. The basic research supported by Tawfik’s RAF award has already yielded significant findings that are taking pain research in unexpected directions. Microglia have long been implicated as primarily detrimental, which suggested that eliminating them from the site of an injury could provide pain relief. “Our initial hypothesis was that by killing these problematic microglia, we would improve pain,” she says. “But it didn’t work. It was only when newly born microglia with a sort of pro-resolution or anti-pain phenotype repopulated the nervous system that pain behaviors improved. It was very different from what we expected. It really lays the groundwork for the potential future development of microglial-targeted therapeutics.”
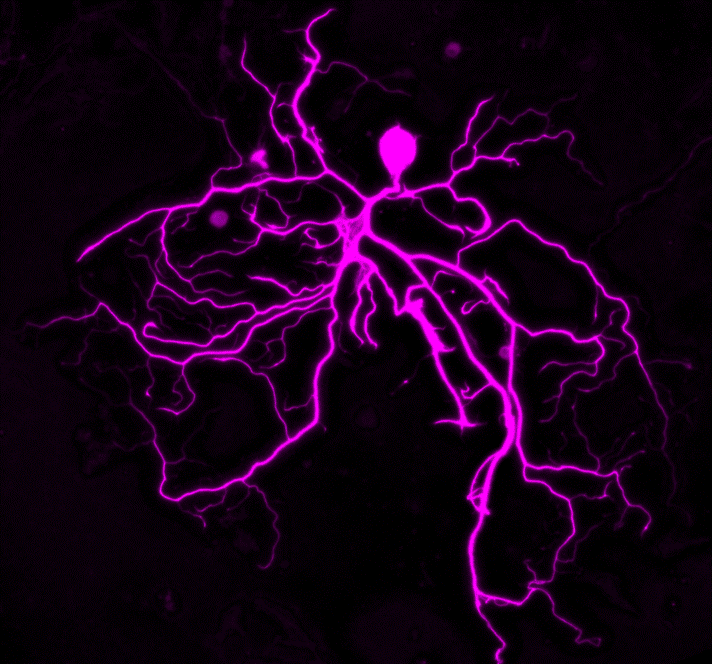
Tawfik shared with us some reflections on how support from the Rita Allen Foundation has accelerated her research—and what she would like more people to understand about pain itself.
What impact did getting an Award in Pain have on your work?
It’s been career-changing. It’s gratifying to receive recognition that your ideas have substance and are worth supporting. And the award money gives you the resources to be able to build preliminary data that ultimately can go into a first major grant application. I used my award proposal—and the data I gathered while supported by the award—as the basis for my first R-level application. And it was a success.
Is there something you wish more people knew about pain?
Pain is a common experience, but because nobody dies from pain—they die in pain—it can be hard to rally people around solving the problem of physical suffering. We’re not going to make significant clinical progress until we understand more about the mechanisms underlying chronic pain and how they may differ depending on the type of pain. Somebody who comes in to see me with migraine does not need the same treatment as somebody who’s coming in with back pain or somebody who’s coming in with complex regional pain syndrome. And I want to be able to appropriately tailor treatment to each one.