
Stories
Ye Zheng: Taking Risks to Understand Regulatory T Cells

2010 Rita Allen Scholar Ye Zheng’s career in immunology was sparked by action and reaction. During his childhood in China, he was encouraged to explore science by his parents who were both engineers—his father an electronic engineer, and his mother an automation engineer. He followed their advice and discovered a strong aptitude for chemistry thanks to a high school teacher who introduced him to the scientific method and experimentation.
Zheng recalls, “I was just fascinated with all these different elements. That they can attract each other, and when you put them in liquids together, a reaction occurs—a precipitation forms, or bubbles, or the color changes. I always was fascinated by this, and that’s probably the first time I really wanted to actually do some kind of research.”
Zheng’s interest and talent in chemistry led him to represent his high school as part of a Chemistry Olympiad competition during his senior year. It was a wonderful experience for him that opened the doors to attending one of the best colleges in China. However, Zheng had already mastered his college-level chemistry requirements while still in high school. This left him with a difficult decision: should he continue to study chemistry, or should he move on to something new. Without hesitation, Zheng decided it was time to move on to something new. He majored in biochemistry and molecular biology at Peking University (Beijing, China); an exciting and new field for him that also had some connections to his roots in chemistry.
On his path to a research career, Zheng attended Columbia University and received his Ph.D. in immunology, followed by a post-doctoral fellowship at the University of Washington with Alexander Rudensky. In 2009, he moved to the Salk Institute for Biological Studies to start his own lab, where he now explores three major research areas:
Tregs and Wound Healing
The Zheng lab studies a particular population of T cells called regulatory T cells. The main function of Tregs is to suppress the immune response—and when these regulatory cells malfunction, it leads to loss of control of the immune system and autoimmune diseases. “Recently, we made an exciting discovery in my lab; we found a second function of these regulatory T cells by accident. We discovered that when you remove the hair from a knockout mouse, the hair fails to grow back. Typically, in a laboratory mouse, when you remove the hair, in two weeks, the hair grows back. It’s just like there’s no difference, but in this knockout mouse, it is different. And we dug into it. What we found is these regulatory T cells in the skin actually feed into the stem cells in the hair follicles, and that helps the hair to grow. So, the stem cells somehow need a support signal from the regulatory T cells to activate and grow. We think we found a second function for Tregs. In addition to immune suppression, they might actually support tissue stem cells for regeneration or wound healing,” explains Zheng.
T cells and Metabolic Diseases
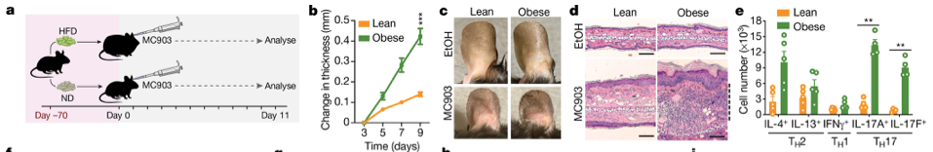
Zheng and his colleagues also study the connection between helper T cells and metabolic diseases—exploring how obesity and other metabolic disorders can impact the immune system. In a recent study published in Nature, the Zheng lab observed how lean and obese mice responded to skin inflammation induced by a Vitamin D-like compound, MC903 (image a). The team observed that the obese mice had a much more pronounced inflammatory response, as indicated by ear thickness (image b-d). In addition, researchers noticed that the T cell populations and the signaling proteins, or cytokines, they secret differed between the lean and obese mice (image e). Zheng and his team observed a different underlying inflammatory mechanism for the same disease in lean and obese mice, and later in the study, the team also noticed this difference translated into how the mice responded to treatments. These results indicate that precision medicine, or customized treatments, may offer a new avenue of care for patients who are managing metabolic disorders that can increase the risk of heart disease and stroke, as well as those affected by immune disorders such as diabetes, rheumatoid arthritis, and lupus. In summary, the impacts of these metabolic disorders are deeper than just the physiological changes observed, the underlying biology of disease processes and response to treatment are also affected, and conventional treatments may not work as intended in these patients. There is no one size fits all.
Treg Stability and Function
“Immune cells attack viruses and bacteria, but the regulatory T cells suppress the immune response. So, when there is a problem with regulatory T cells, then the immune system basically starts to attack our own tissues and cause autoimmune diseases. For example, in Type 1 diabetes, our immune system kills the insulin-producing beta cells in the pancreas leading to a long-term, chronic illness. So, regulatory T cells are really important to contain and prevent this kind of harmful immune response,” explains Zheng. From this observation, one key question the Zheng lab addresses is how Tregs can balance between a helpful and harmful state while responding to environmental conditions. His lab has examined the role of a transcription factor, or regulatory protein, Foxp3, in Treg cell lineage development. When the Foxp3 gene is “on” it is continuously expressed in the Tregs and maintains their suppressive function in the immune system, but if this expression is lost, or Foxp3 is turned “off”, Tregs become ineffective at controlling the immune response, leading to autoimmune disorders. Zheng and his team are mapping the pathway for Foxp3 in Tregs to understand how it changes in these cells under different conditions. This work is important for developing autoimmune disease treatment and cancer immunotherapy.
“Recently we made an exciting discovery in my lab; we found a second function of these regulatory T cells, by accident. We think we found a second function for Tregs. In addition to immune suppression, they might actually support tissue stem cells for regeneration or wound healing.”
Zheng is now an associate professor at the NOMIS Center for Immunobiology and Microbial Pathogenesis at Salk. Here, he reflects on the impact of the award and his career trajectory, as well as the impact of emerging technology on the future of research.
Can you speak a little bit about how the award has influenced your research and career trajectory?
What the Rita Allen Foundation did is offer the longest support and the biggest support in financial terms, and that actually gave me the confidence to take more risks when I started. I could do another project instead of just one project. I could spread my wings a little bit more so that, I could go a little bit farther. It made a huge difference in terms of my risk-taking behavior. But, also, I think just the interaction between the Scholars community—we had meetings and we all helped each other—that is very stimulating, as well.
What subsequent research and publications came out of the funding you were provided by the Rita Allen Foundation?
So, the research proposal I proposed at the very beginning for the Foundation, I abandoned within two years because it was not productive. So, I think in my first 5 or 6 years, I was able to produce a Cell paper and a Nature paper, and these were both kind of high-risk research. I cannot say that the Foundation’s support was the only support I had for those years, but definitely it’s one of the major factors that made me willing to take more risks to get more work from that period.
How has emerging science and technology impacted your approach to research and what are you excited about looking ahead?
I feel that because of CRISPR and related technologies, it’s really an exciting time for immunologists. We can take blood cells, do some engineering, and then put them back into the body to perform a new function. So, immune cells can be tailor-made to do a variety of tasks. Multiple companies are currently trying to design Tregs to treat autoimmune diseases, and there are some pretty good successful cases in arthritis and Type 1 diabetes. So, I think, this engineering side of biology is going to be the future.
What are things that interest you, away from research?
I’m really into outdoor exploration, like hiking and backpacking. Before, in the summers, we’d go hiking or camping every weekend. That changed when I started my own lab. I just don’t have as much time. And, then I had my kids; it was just hard to carry the little ones to do a long hike in the wilderness. Now that they have grown up a little bit more, both of them like to go outdoors. So, I’m now getting back into hiking and camping with my kids.