
Stories
Samara Reck-Peterson: Research in Motion—Visualizing and Connecting Motor Proteins to Disease

Samara Reck-Peterson’s research focuses on molecular machines—the macromolecules that transport intercellular compartments. Her work reveals how these life-essential mechanisms operate at molecular, cellular, and organismal scales. As a 2009 Rita Allen Scholar, she found the freedom to be creative in her research—exploring connections between “the cellular interstate system” and human diseases such as Parkinson’s—and to take risks in pursuit of discovery.
That creative energy continues to fuel her highly collaborative and interdisciplinary research and to inspire her team’s recent work investigating the functionality of molecular machines in other life forms, such as cephalopods. “It turns out that intelligent cephalopods—specifically, squid, octopus, and cuttlefish—do something that’s fairly unique. They edit their messenger RNAs, and those edits sometimes lead to changes in the amino acid sequence of the downstream protein—a process that is called recoding. And it turns out that the proteins that power the mechanism we study—intercellular transport—are really highly recoded in these organisms.” Sparked by curiosity, Reck-Peterson’s team began to think about this unique recoding and the extreme temperature environments these animals live in.
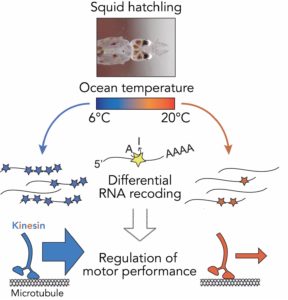
Through a collaboration with the Scripps Institute for Oceanography, Reck-Peterson and her lab team conducted an experiment with cephalopod hatchlings, placing them in different temperature environments and then isolating and sequencing their mRNAs, looking for the impacts of recoding. “We found that there is a lot more recoding in cold environments. We could then recombinantly make the proteins that would mimic the actual recoded proteins and test their motile properties by watching the movement of individual motor proteins using light microscopy. And what we found is the motors that had been recoded behaved much better in the cold than the motors that hadn’t been recoded. This supports our hypothesis that these animals are recoding in order to enhance the performance of these machines at different ocean temperatures,” explains Reck-Peterson. These kinds of interesting and creative experiments give insights into how animals that live in extreme conditions are able to adapt to their environments at the molecular level.
Before Reck-Peterson explored recoding in cephalopods, she grew up in an environment that nurtured curiosity. Her parents instilled in her a wonder about the world, and as she continued in her studies from undergraduate to graduate school, Reck-Peterson was also supported by great mentors—Susan Singer at Carleton College, Mark Mooseker at Yale University, and Ron Vale at University of California, San Francisco. “At various times, I thought about heading in different directions with my career, but these mentors have really been the ones that have helped me stick to my goals of making discoveries in science and biology,” says Reck-Peterson.
After graduating with a B.A. in biology from Carleton College, Reck-Peterson was drawn into the world of motor protein research. She attended the physiology course at the Marine Biological Laboratories in Woods Hole, taught by Mooseker—who was studying myosin motors, which move along actin filaments allowing for cellular movement. Reck-Peterson recalls, “I remember one of the things we were doing in the course was purifying a myosin protein called myosin V, a cargo-carrying isoform of myosin, from the brains of chicks. I think for me, just this idea that you could purify these machines from cells, then watch them move, and try to understand their mechanics was completely fascinating.”
Reck-Peterson went on to complete her doctorate in the Mooseker lab, studying a type of myosin that is responsible for movements inside the cell. Afterward, she continued to investigate molecular motors as a postdoctoral fellow in Ron Vale’s lab at the University of California, San Francisco, and focused on a different type of motor that travels along microtubules called dynein.
While a postdoctoral fellow, Reck-Peterson was also encouraged to think about ways she could contribute to the scientific community around her. “I spent some time thinking about different programs that we could start for postdoctoral fellows at UCSF. And one of the things we started at the time was a research-in-progress seminar series where graduate students and post-docs present their latest findings. We also started an award for postdoctoral research to recognize the contributions of post-docs. And I helped develop a lab management course with Bill Lindstaedt, who ran the career center at UCSF.
“I think that being a Rita Allen Scholar, and later being recognized by the NIH for the New Innovator Award, and more recently by HHMI as an Investigator, has allowed me to be creative and to take risks in my science.”
After completing her postdoctoral fellowship, Reck-Peterson joined Harvard Medical School in 2007 as an assistant professor, and in 2009 she was selected as a Rita Allen Foundation Scholar. Now, a professor at the University of California, San Diego and a Howard Hughes Medical Institute Investigator, she continues to apply her creativity and curiosity to the fascinating world of molecular motors—engaging in highly interdisciplinary research that utilizes in vitro biochemical reconstitution, protein engineering, single-molecule imaging, proteomics, live-cell imaging, and genetics to achieve her team’s goals.
Reck-Peterson’s lab also studies a protein called LRRK2, whose gene is one of the most commonly mutated genes in Parkinson’s disease. She explains, “Working with our collaborators, Andres Leschziner (Department of Cellular and Molecular Medicine, UC San Diego) and Elizabeth Villa (Department of Molecular Biology, UC San Diego and a Howard Hughes Medical Institute Investigator), we discovered that the LRRK2 protein can bind directly to the microtubule tracks that my lab has been studying for so many years. We showed that the LRRK2 protein forms filaments around microtubules both in cells and when removed from cells. Those filaments can act as what we think are physical barriers for the motors that move along microtubules. Drugs that target LRRK2 enhance this association, making it important to understand the physiological role of LRRK2’s interaction with microtubules.
“Interdisciplinary science is the future of science. As technology improves, it’s hard to be an expert at everything, so you have to be collaborative to answer the questions you want to answer. This is really a guiding principle for me in my research program.”
In another interdisciplinary collaboration—one with surprising results—the Reck-Peterson Lab partnered with Matt Daugherty (Department of Molecular Biology, UC San Diego) to understand how the transport machinery interacts with viruses. In this project, Reck-Peterson and Daugherty are exploring how viruses use molecular motors to their advantage. Molecular motors use tethers, or adaptor proteins, to attach to and transport larger structures in the cell. The Daugherty lab showed that one of these adaptors has evolutionary signatures of interacting with a pathogen. The initial hypothesis for this work proposed if the adaptor protein was knocked out, it would inhibit viruses from successfully infecting cells. However, the results from experiments showed the complete opposite—the viruses thrived. Even more interesting, when these cells were also treated with interferon to help mount an immune response against the viruses, the cells remained unresponsive, and the viruses continued to thrive. How might viruses knock out the adaptor? Reck-Peterson and Daugherty showed that the adaptor was a target of some viral proteases. “Viruses like SARS-CoV-2 make a protease that is used to cut their viral polyprotein apart. That same protease can cleave the adaptor protein. So now, our hypothesis is that if the viral protease has cut this adapter, somehow that is shutting down the interferon response,” she explains.
Here, Reck-Peterson discusses her interdisciplinary collaborations, the process of becoming a Scholar, and interests outside of the lab.
What inspires you to be so highly interdisciplinary in your approach?
Interdisciplinary science is the future of science. As technology improves, it’s hard to be an expert at everything, so you have to be collaborative to answer the questions you want to answer. This is really a guiding principle for me in my research program. I was influenced by my time as a postdoctoral fellow at UCSF, which was a really collaborative place. The environment, even the layout of the building I worked in, was set up in a way to encourage collaboration. That influenced me and was something I wanted to try to replicate in my own lab.
What do you remember about the process of becoming a Rita Allen Foundation Scholar?
I remember being nervous, but then when I got into the room, as soon as I started talking about my dreams as a scientist, I immediately wasn’t nervous anymore. I think that being a Rita Allen Scholar, and later being recognized by the NIH for the New Innovator Award, and more recently by HHMI as an Investigator, has allowed me to be creative and to take risks in my science.
Would you share something you enjoy doing outside the lab?
I love gardening. I moved to southern California seven years ago from Boston. Because I can now garden all year round, it’s been fun to figure out what grows here at different times of the year. Right now, I’ve been enjoying growing different types of hot peppers and making different types of homemade hot sauce out of those.