
Stories
Emmanuelle Passegué: Seeking the Secrets of Blood Stem Cells
Emmanuelle Passegué had a rather unglamorous introduction to research as a young college student in France. Initially attracted to the field by fascinating papers filled with beautiful images of clean, unambiguous molecular mechanisms, the realities of working with fruit flies for biological research turned out to be quite different.
“From classwork I thought, ‘Oh, this is fantastic! That’s the biology I want to do,’” Passegué recalls. “And then you end up behind the microscope, with a petri dish full of crawling larvae, and your job is to separate the larvae, cull the larvae, and so forth. This literally repulsed me. So I thought, ‘Let’s try to find something that fits more with my personality.’” Very quickly thereafter, Passegué realized that working with mammalian experimental models—such as mammalian cell culture, rats, and mice—suited her much better and was even appealing.

Emmanuelle Passegué (Photo: Courtesy of Emmanuelle Passegué )
Despite the ups and downs along the way, Passegué never lost her initial fascination with research, sparked and nurtured by her undergraduate biology teacher, Monsieur Poisson. She remembers, “M. Poisson had a research lab at the university and was really passionate. That was the first time I had an instructor teaching me biology and also really living it and involved in doing research. That drove me to specialize in the biology track during my university years. And as soon as I could during my last year of university I jumped at the opportunity to work in a laboratory. For the first time, I could fully see what an experimental research approach was—where you actually think about doing an experiment, encounter all the obstacles, watch the results emerge, and draw conclusions from the results. I loved it from the get-go.”
After college, Passegué went on to pursue a doctorate in endocrinology at the University of Paris XI. Up to the point of graduation, she was contentedly completing her training, but when it came time to make a decision about what to do next, Passegué found herself facing an unexpected moment of uncertainty.
“I will always remember this conversation with my graduate mentor, who asked me, ‘What do you want to learn? What do you want to do next?’ And I didn’t know. I really didn’t know. I found that interesting. I liked this. I thought that could be a nice area of research. Every time, my graduate mentor told me, ‘This is too vague; that is not precise. If you want to study the cell cycle, this is much better. Do you want to work on that model?’ It was a moment where I felt I was not in control. I didn’t know how to make a choice. So I did what I am now seeing a lot of graduate students and undergraduate trainees doing—I went with the best, shiny new technology of the moment.”
At that time, it was mouse genetics—creating genetically altered mice in order to better understand genetic functions in the human body. As a postdoc, Passegué joined the lab of Erwin Wagner in Austria, where they were implementing mouse genetic technology, focusing on the AP-1 family of transcription factors, Fos and Jun. Passegué worked on JunB and cFos as a transcription factor complex, which regulates gene expression in cells. It was interesting work but it only become fascinating when one of the mice she was working with started developing a blood malignancy, chronic myelogenous-like leukemia.
“This was really wow,” she recalls. “I loved the question! I started reading textbooks, everything I could find—what are myeloid cells, how are the hematopoietic lineages being produced, what’s their function—and getting into hematopoietic stem cell biology. I read a lot of papers on this topic by Irving Weissman at Stanford University, and basically just tried to copy what they were doing in his laboratory. I immediately saw that after my first postdoc, I wanted to get trained in the field of HSC and leukemia biology.”
Passegué continued her training with Weissman, who was well known for his pioneering work on hematopoietic stem cells—the stem cells in bone marrow that produce blood cells—and hematopoiesis under normal and malignant conditions. She completed this second postdoc in 2005, establishing her path to becoming an independent scientist. The same year, she set up her own research lab at the University of California, San Francisco, continuing to study hematopoietic stem cells and hematopoiesis as part of the California Institute of Regenerative Medicine research consortium. In 2008, she was selected as a Rita Allen Foundation Scholar.
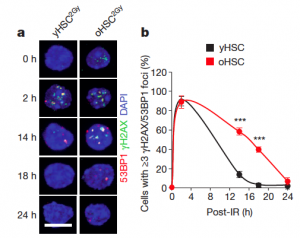
Rita Allen Scholar Emmanuelle Passegué and her team research aging. In a recent study, the Passegué lab examined the functional decline in haematopoietic stem cells with aging. HSCs collected from young (6 -12 weeks, yHSC 2GY) and old (22 – 30 moths, oHSC 2GY) mice were treated with ionizing radiation (2Gy), which causes DNA damage. The cells were stained for DNA strand breaks (53BP1, red) and DNA damage response proteins (gamma H2AX, green) located in cell nuclei (DAPI, blue) to determine DNA repair kinetics. Overall, oHSC have slower repair kinetics compared to yHSC (b, right). Examining the response to DNA damage gives researchers understanding of how the blood system declines with age and identifies potential targets for rejuvenation therapy to counteract this vulnerability. (Image: Flach et. al. [2014] Nature)
In 2017, after 12 years at UCSF, Passegué relocated her lab to Columbia University, where she is now a Professor of Genetics and Development with an Alumni endowed chair, and the Director of the Columbia Stem Cell Initiative.
“If you understand the stress-response wiring of the system, then you can understand what goes wrong with disease, development, or aging.”
Here, Passegué reflects on the process of becoming a Scholar, the challenges and rewards of her research, and her role as Director of the Columbia Stem Cell Initiative.
What do you remember about the process of becoming a Rita Allen Foundation Scholar?
I remember being very, very impressed and terrified by the interview. This was my first time actually coming to present myself and defend my research. It was prestigious, and we were all in competition with each other. We were all together for this interview day—passing one another, everybody looked very stressed, and I was very stressed.
I think the value of foundations like Rita Allen cannot be underestimated. It’s absolutely fantastic to receive this kind of career development award that allows you to develop yourself as a scientist—and to do that at a young stage, when you’re starting your lab, is key, absolutely key.
What excites you about your work?
I’m really fascinated by biological mechanisms of stem cell control, like metabolic activation. I think this is a fundamental feature of how you turn a quiescent (inactive) stem cell to an activated stem cell that will make decisions on cell fate. So we study many stress-response mechanisms, how they control the activity of various stem and progenitor cells in a constantly changing microenvironment, and what are the pathways that dictate when the cells will survive, will die, and will undergo certain types of differentiation.
We also look at how you activate pathways of regeneration like myeloid regeneration—this has always been the focus of my research in hematopoietic stem cells. I am fascinated by how you turn a very immature, non-specified and rare stem cell population into this abundance of very diverse and complex myeloid subtypes.
It’s such a complicated and interesting field. If you can control activation and quiescence in the hematopoietic stem cells, you can really modulate the rate of blood production. When you know the mechanisms that trigger certain differentiation pathways, you can modulate what type of blood cells are being produced. If you understand the stress-response wiring of the system, then you can understand what goes wrong with disease, development, or aging.
What are the challenges?
The fact that we still cannot image very well the stem cells and the niche—or surrounding environment—because we are working within the most opaque tissue in the body. You have to cut through a rigid bone shell to get to a very gooey bone marrow, and that offers a huge technical challenge to being able to really look at how cells react, behave, change, evolve, interact, and respond within the environment.
The fact that these cells are so rare—you get very few stem cells, very few progenitors, and you cannot do any real biochemistry. You cannot do metabolomics. You cannot do certain types of analyses, so there is a big limitation for the technologies that you can employ in the field.
How, in your role as Director of the Columbia Stem Cell Initiative, do you help to advance research in your field?
I joined UCSF when I started my lab because I felt it was the best place for me at that moment in my career—a nurturing environment, in California, with lots of people doing stem cell research. And it turned out to be an excellent and very successful environment for me. Ten years later, my goals are slightly different. I started to want to exert an influence on the bigger questions: Who do we recruit? Where do we make investments in technology? Where shall we really focus? Where is there a way of pushing the field and pushing discovery by teaming up and getting resources together?
When I started looking around and expressing a desire to have an impact in shaping a community, suddenly the opportunity to take a leadership position appeared, and I got approached to interview at several places with Columbia being one of those places that contacted me. They explained that they had a stem cell program, the Columbia Stem Cell Initiative, which was started in 2008. The program had been leaderless for a couple of years, and they felt that it needed new energy, new direction, and someone who could come with a vision to clearly bring together the stem cell community at Columbia.
I met with the faculty and the leadership, and I found that it was a perfect place for me to get to know what it is to direct an institute with an exciting group of scientists—that really made me excited to come to Columbia. I think I needed that. It was a jolt that made me rethink a lot of things for my own research, reenergized me and pushed my lab in different directions. It’s also the pleasure of getting people together, shaping the community, and helping others, which is what I wanted to do. It’s of course way more work than I anticipated.
Just like when you are starting a new postdoc, you have to learn a lot of new things: new techniques, new environments, new systems. So at my level, this is the equivalent of doing a postdoc in a different country. There’s the same level of novelty, excitement, and pleasure of doing something different.